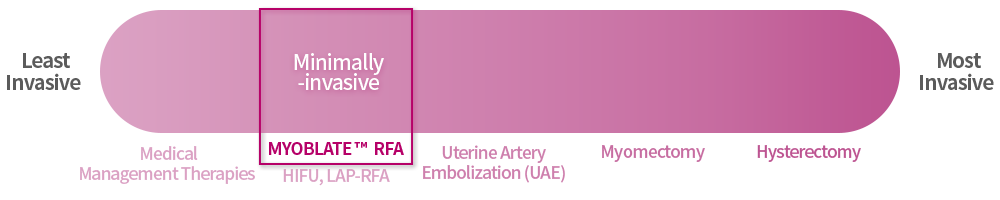
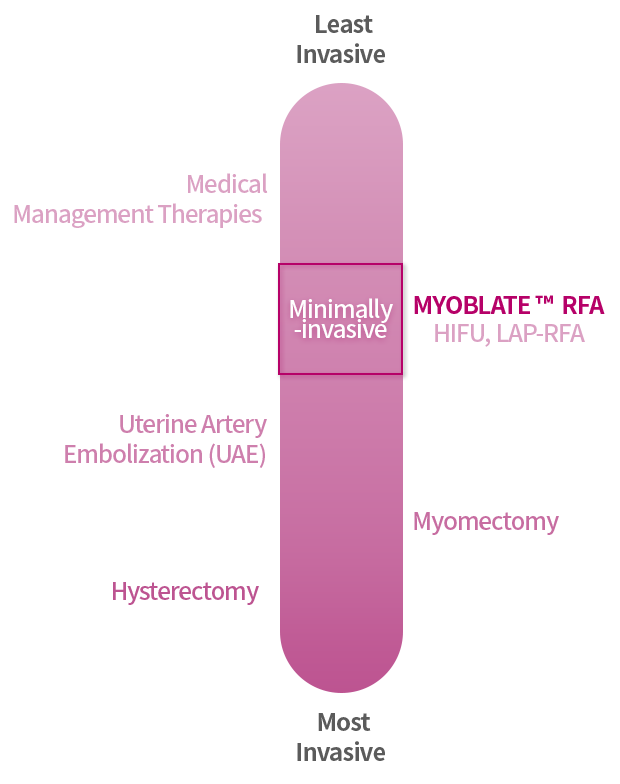
There are different kinds of uterine fibroid treatments available, with surgery (hysterectomy and myomectomy) being the most aggressive and medical management therapies (birth control, IUD, tranexamic acid, Gn-RH) being the least invasive approaches for symptom management.
Treatment Options
What treatment options are available?
Novel therapeutic options include a non-invasive approach that preserves your uterus,
requires no incision, and is done on an outpatient basis.
Novel therapeutic options include a non-invasive approach that preserves your uterus, requires no incision, and is done on an outpatient basis.
What is the MYOBLATE™ RFA Procedure?
Radiofrequency ablation (RFA) is a minimally-invasive procedure that uses heat derived from high-current energy waves to safely treat fibroids one by one.
RFA is not a new concept. There have been other RFA treatments available, such as transcervical RFA and laparoscopic RFA. Therefore, years of RFA treatment demonstrate its efficacy and safety.
While RFA for Uterine Fibroids is not new, the MYOBLATE™ RFA is novel in that it can be used across a range of approaches. This allows for a low-risk rate and excellent clinical outcomes for the patient and her quality of life.
The system for MYOBLATE™ RFA consists of the MYGEN™ RF generator and MYOBLATE™ electrodes, which are CE-certified and indicated for the treatment of uterine fibroids. The V-Guide, a non-conductive electrode guide, allows the electrode to be inserted parallel to the ultrasound, facilitating precise targeting of the lesions by ensuring that the electrode remains within the ultrasound view.
MYOBLATE™ RFA for fibroids is performed in a simple way with the guidance of a transvaginal ultrasound probe. After locating the fibroid, the physician may begin heating the fibroid using the RF electrode attached to the ultrasound probe.
Procedure steps
STEP 1
The physician uses the ultrasound system to confirm the size and location of the uterine fibroid(s).
STEP 2
After detecting the fibroids, the MYOBLATE™ RF electrode is attached to the transvaginal ultrasound probe using V-guide and inserted through the vaginal canal.
STEP 3
The MYOBLATE™ RF electrode is safely placed into the uterine fibroid and radiofrequency energy is delivered to treat it.
STEP 4
After confirming that all fibroids are treated, the electrode is removed. Patients are normally released within a few hours, and experience progressive symptom relief and continual improvement over time.
Depending on the type of fibroids and preferences, the procedure can be done
by transcervical approach with abdominal ultrasound guidance.
Depending on the type of fibroids and preferences, the procedure can be done by transcervical approach with abdominal ultrasound guidance.
How does Radiofrequency Ablation work?
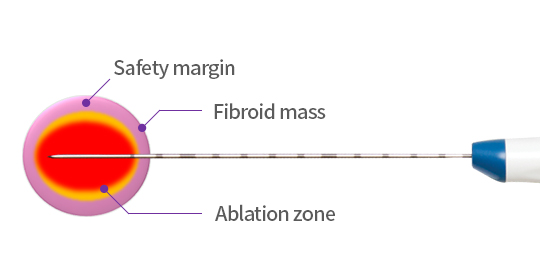
01
The Generator creates RF energy that is safely delivered to the MYOBLATE™ RF Electrode (sometimes called Needle). The heat dispersed at the electrode’s tip destroys the fibroid tissue. It only ablates 70-80% of the fibroid mass to preserve healthy uterine tissue.
02
By causing coagulative necrosis of the fibroid tissue during the procedure, the denatured cells are dissolved over time. Symptoms are resolved gradually, and the uterine fibroid stops growing. After a year, the fibroid generally shrinks by 60–90%.1-3
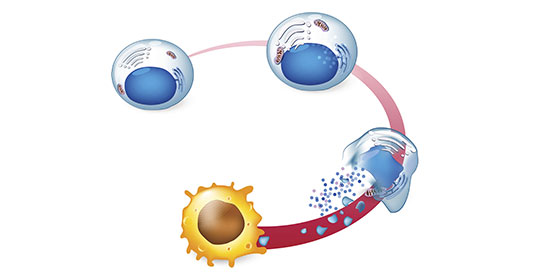
Advantages of the MYOBLATE ™ RFA Procedure

Well-tolerated and Clinically Effective
Transvaginal, transcervical, or laparoscopic approaches Minimally-invasive, no incision, with short procedure time.
Targeted Approach
Ultrasound and a needle guide are used to accurately target fibroids, destroying only the fibroid tissue.

Outpatient Procedure 1,5
Fast recovery and return to normal activities. Conscious sedation can be used to exclude the risk of general anesthesia.
Patients generally return to their normal activities within 24 hours of the procedure.

Cost-Effective
MYOBLATE ™ RFA requires lower healthcare expenses compared to other treatments as it is an outpatient procedure and a hospital stay is not needed.
Clinical Benefits

MYOBLATE™
Since the uterine fibroids are treated from the inside during the MYOBLATE ™ procedure, no scars are formed, and uterus can be preserved.
RFA
RFA for uterine fibroids targets only the fibroid, resulting in the immediate cessation of associated symptoms, preventing the fibroid from growing to a problematic size, and possible volumetric shrinkage of the fibroid.

Symptoms relief 1,4
Fibroid-related symptoms are reduced.
Symptoms such as heavy bleeding
or discomfort resolve quickly.

Uterus Preservation 1,2
Uterus preservation with minimum damage
Stop the growth
and fibroid shrinkage
Due to the coagulative necrosis of
fibroid cells by thermal ablation.

No scar
By transvaginal approach, the MYOBLATE™ electrode is inserted through the vaginal canal, so it does not require any incision or puncture.
Low complication rate 3,5
Studies with follow-ups show that
RFA is just as safe as other treatment options.

Quality-of-life improvement 4,5
Symptom relief, with no need for
medicine for the rest of one’s life.
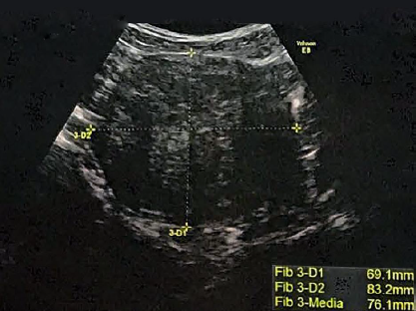
Before
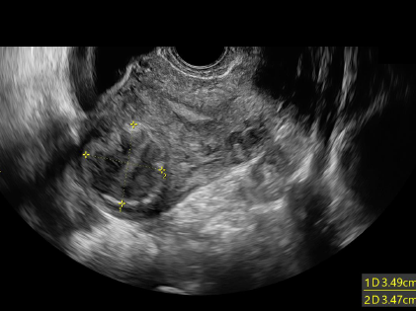
After 12months
What results can be expected?
The primary goal of radiofrequency fibroid treatment is to stop fibroid growth and relieve patient symptoms. In most cases, however, we can also expect a reduction in fibroid volume. In general, three months after radiofrequency treatment, the uterine fibroid volume may reduce by 60-90 percent.2
It is important to keep in mind that fibroids may re-grow and new fibroids may be reduced by after treatment. Radiofrequency ablation for uterine fibroids is a minimally-invasive procedure, and complications and recovery time are minimized.
LEGAL DISCLAIMER
Last Updated August 9, 2022
IMPORTANT SAFETY INFORMATION
The MYOBLATE™ electrodes are CE-certified and indicated for the treatment of uterine fibroids. The content herein is solely for informational and educational purposes and should not be construed as medical or health advice. While the MYOBLATE™ Uterine Fibroid RFA Procedure may offer substantial benefits to many patients, it is not universally applicable, and individual outcomes may vary. Please contact your medical professional for specific advice regarding the potential benefits, risks, and suitability of this treatment in your specific case.
LEGAL DISCLAIMER
This information is not intended as an offer for sale or promotion in jurisdictions where such activities are restricted. The imagery used in this material is for illustrative purposes only, featuring models, and should not be interpreted as an endorsement or representation of typical patient outcomes. For more details about information provided on this Site, please click here.

